

How to Use Digital Technologies to Retain Clinical Trial Participants
Only one in ten drugs that are developed — despite the amount of money invested in the development and clinical trials — gets FDA approval.
Considering the fact that full-cycle drug development takes about seven and a half years, and its cost reaches $2 billion, the 1 in 10 stats is a plain failure, and it’s hitting the industry hard.
What impacts clinical trials success?
If we want to come up with a solution to fix the failure situation — and use digital technologies as assets — we need to understand why it occurs.
Clinical Development Success Rates report reflects on wins and fails in pharmaceutical research and development during 2006–2015 years (the case haven’t changed much since then). What have the authors found?
1. Clinical validation is long and it varies. Clinical validation is long for different classes of drugs, drug targets, and drug action mechanisms. The table is pretty explanatory:
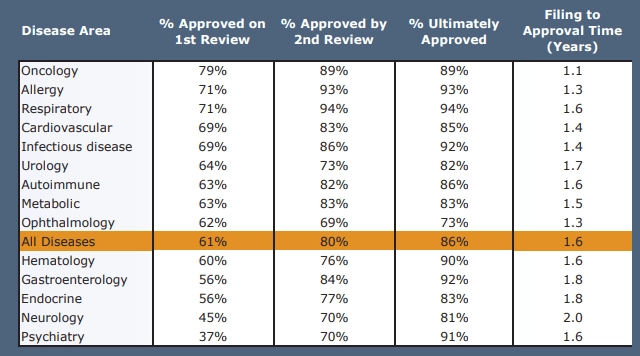
2. Investigative drugs to treat chronic and high-prevalence diseases require more time and additional studies — and, therefore, more efforts to retain and engage their participants.
3. Usage of biomarkers increases the likelihood of FDA approval. Detailed, throughout targeting of the further trial participants, is one of the significant parts of a trial success — that includes the usage of selection biomarkers as inclusion/exclusion criteria, characteristics of human body researchers can measure (for instance, blood pressure, or a total kidney volume).
In clinical trials, biomarkers become metrics, a “report” on drug efficiency. Researchers can detect a biomarker signature connected to a drug response — or, to aim the drug at some biomarker to see how it reacts. Then, on the basis of this preliminary data, the first stages of clinical trials are designed and CROs invite the parts of the population who are more likely to benefit from a certain drug because of their biomarker fit.
That, among obvious value-based medicine reasons, allows pharmaceutical companies to lower the cost of failure.
Other, non-clinical reasons for failure are competition between different research programs in an organization and lack of funding.
Let’s summarize:
- Precision is vital for trials from the drug development planning and, to increase the likelihood of success, tools to enhance it must be incorporated from the start. But this is not the case for most of the trials.
- Clinical trials are VERY. LONG.
- Sometimes, they are longer than initially anticipated.
What does all of this mean for perspective further participants?
It means trials require a lot of commitment, and these tons of commitment don’t guarantee finding a successful treatment. And that leads us to one of the main pains for CROs:
Sad clinical trial recruitment leaky funnel
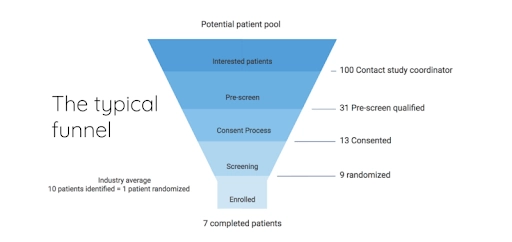
Last year, researchers from the Center for Information and Study on Clinical Research Participation figured : while the majority of respondents understand the significance of clinical trials, they know little about what they are and perceive the opportunity to participate as a burden.
Low level of awareness in combination with factors for research success we’ve listed above results in the following: almost 70% of respondents “never or rarely considered” clinical study as a treatment option, especially residents of Europe of Asia Pacific region.
In addition, while more than half of the CISCRP’s survey respondents think it’s best to get to know about clinical trial participation opportunity from their doctors, only few participants were addressed with the matter by their physicians.
What does a potential recruit wanna know about a clinical trial?
- Risks and benefits.
- Study’s objectives.
- Types of medical procedures.
- If there are confidentiality protection measures.
- Location of the site, lab, clinics.
- Costs and reimbursements.
- Lengths of the study.
- Receiving a study summary/results.
- Getting “supporting study info.”
- Getting info on health management.
Considering the fact, most of the potential recruits from the top of the funnel get AWOL after viewing consent form, CROs are yet to achieve clarity and understanding in communication with them.

As for getting participants to complete the trial, CROs should take into account other things, such as timely compensation, educational information about participants’ condition, scientific knowledge about a drug, and a good relationship with them. Often, people want to keep their promise to complete the trial and they always, obviously, want to feel the effects of treatment — to help them achieve that, CROs have to engage them, talk to them, in other words — retain them.
Your digital clinical trial participants retention strategy
So, subjects leave. Why? Because they fear something will go wrong. Or because the family needs them. Or because they feel like that.
Let’s start it backwards: before designing a clinical study, you should figure out what obstacles your potential subjects may face while they’re on protocols. Picture smartphones, apps, and other cool technical stuff as a way to close gaps between researchers and participants before they occur — or as a method to address them hands-on, quickly, preventing a dropout.
Before going in the digital world, formulate Informed Consent Form and test it among other people who are not related to science in any way. You’ll find a lot of insights from out there. Be as clear and concise as possible, explain complex terms, aim to awaken curiosity instead of confusion and feeling of being dumb.
It’s also possible to adopt e-consents that makes consent process more simple, and even fun and interactive. Which is good for the integrity of your funnel.
Shortly on building software around humans
Now, about that digital tech. Martin Weigel from Wieden+Kennedy Amsterdam once said that advertising is very incidental to real life, and the real challenge for creativity isn’t about growing enthusiasm to buy (or participate in, in our case, yeah?) something, but to overcome indifference.
You need to build software — whatever device you’re planning to use — around people and the fact that they, as a rule, have many interesting things to do, a life to live. Of course, there is money. But you’ve seen stats. 7/100 people. Are they motivated by money? Perhaps some of them. But also they’re motivated by an opportunity to get better. To make an impact. By their curiosity, wit, and interest in science.
So, figure out their demand and create something that’ll satisfy it— and, at the same time, that will mitigate their pains.
One. Talk to them and let them talk to each other
Constant communication is something all of us get used to — but large businesses from serious industries are still struggling to employ digital talk in their processes. If you build an app, a platform, or any other kind of HIPAA-protected software your subjects are supposed to use, make sure you have a team that is ready to answer their urgent questions, worries, and complains (bots can help them handle requests).
Employ participant support — through chats, calls, telemedicine. It’s like customer support. You have to build a people to people relationship within your trial — it is, according to this study, one of the things people expect from involvement in trials. They want to feel like a part of the science community, part of a movement that pushes medical advancement forward. They are also very anxious — and your specialists must have a way to soothe and calm them, because, as you know, when people fear side effects strongly there’s a large probability that sooner or later they will feel something that will look like side-effects.
You should monitor those things — and nurture trust through being accessible, open and clear. Try using telemedicine even if you’re working in cities — elderly people, as well as Millennials and Gen Z, prefer to stay home instead of going to consultation — and still get an answer to their questions. And most of your potential participants are located two hours away from study centres if you’re operating in America.
Include chats, forums, and other places for group talks. If you’ll create an app with a possibility to talk anonymously to other participants of the study, that will, too, encourage a feeling of companionship. A good idea is to engage your users in group activities — tests, games, quizzes. Collaboration with peers may help deal with stress.
You can create educational games — with a simple script that relates to study, or create a platform where you’ll be publishing — in layman’s terms —articles about how the study proceed (avoiding confidential stuff) or posts on related scientific and medical news.
You can ask them to share their stories about how they've decided to participate. By the way, it’s very useful if you’re testing drugs for, for instance, chronic illnesses management: sharing complicated experiences in a non-judgemental environment help people bond and deal with accepting what’s going on with their bodies. Of course, these interactions should be anonymous as well to protect patients’ privacy.
Appreciate their input. Appreciation may be sent via message with kind words, in form of gamification, in a letter, in a bonus, etc. Make sure you’re sending words of thank you — especially on the first stages of participant selection when they have to go through tests, diagnostics, and other, rather unpleasant and time-consuming things.
Two. Educate and be honest
Include interactive updates on study/participants’ progress. Inform them about the progress of the trial — and don’t lie, if there isn’t any. If you’re having an adaptive trial — make sure your participants know if the study changes after you’ve discovered new evidence. It can be done in an interactive way — video and text updates, a news feed, anyway your subjects prefer to receive news. (Yeah, you’ve got to know this one.)
Visualize complex things. Trials designed in adaptive ways usually use biomarkers, new and old, — to quickly react on what’s going on in participants’ body during the treatment — and to deliver quick insights for managing serious diseases. It’s important for participants to know what’s going on with them, to feel, at least in some way, in control, so try sharing with them the info about processes in their body. If you’re running diagnostics, visualize what’s going on after they receive treatment. Show them changes in biomarkers. Explain, how they reflect their overall condition.
Fight misinformation and fear. We loved a study that included information campaign associated with Ebola, vaccines, and clinical studies where trial designers bust myths about these things during preparation for the trial. They understood: there’s no way to successfully launch and complete trial in the misinformation bubble — so they’ve ruined it through personal sessions, organized by people, potential participants knew and trusted. It worked — and it worked quickly, with high retention rates, which was essential due to the Ebola outbreak situation.
Three. Employ trackers and scheduling tools
Medication adherence is a factor of utmost importance for clinical trials, as well as regular visits to research sites and, in general, commitment. How to drive commitment in the world of endless distractions? By motivating the participant to be persistent in their efforts to achieve their goal (become better, help the medicine, try a new treatment, etc.) through awareness and education and enhanced self-management. Simply said, make trial about them as much as possible.
Develop mood-condition trackers. It takes much more cognitive efforts for people to wait for long-term results of action than to jump to some short-term solution. But any long-term goal consists of a bunch of small steps, so — show it to them. Let your participant track their mood, to reflect on how they feel during a study. Show these data to your employees and give them the opportunity to react if something goes out of the pattern. Better, show this data to tracking algorithm and instruct it to notify researchers if something is out of pattern — that’s the power of automation for you.
Include calendar and reminders. Visualize the timeline of a trial, employ medication calendar and notifications associated with it. Write microcopy: gentle encouragement, motivational quotes (they do help some people), or statistics on how other trial participants are doing. Present participating in a trial as a community effort.
Four. Gather their feedback and connect it to trial outcomes
A nice thing about digital solutions you offer to clinical trial participants is that there is — or there soon will be — a possibility to use all data they generate as source data for the study. Patient-generated reports, for instance, might be concluded from trackers and notes. Wearables may give you additional insights into patients’ state — with proper analytical tools, of course. Using these technologies, you can improve the odds to create a medication that will be more effective , accurate— and you will know why, and for what people, specifically, it works better than for others. When precision medicine will grow out of the growth stage to the stage of “that’s quite common here,” such mechanisms will be very useful for personalized drug development.
Final thoughts - digital technologies to retain clinical trial participants
While it’s understandable that drug development and drug time-to-market suffer from low retention, clinical trial participants have to endure other things. For instance, the fact that traditional clinical trials don’t end even if it’s obvious that the hypothesis isn’t confirmed and the fact studies are often designed in a way that doesn’t consider the needs of participants. If pharmaceutical companies and CROs want to take part in healthcare’s transformation in a service industry, they need to invite patients on board — that will help gain patient-centricity and, at the same time, assist in developing a more in-depth understanding of clinical trial processes in other people.
Tell us about your project
Fill out the form or contact us

Tell us about your project
Thank you
Your submission is received and we will contact you soon
Follow us
